One of the essential concepts in chemistry and analytical science is “What is Beer’s Law”. Simply put, Beer’s Law explains how light interacts with a solution and how this interaction helps determine the solution’s concentration. Also known as the Beer-Lambert Law, this principle is widely used in laboratories, medical diagnostics, environmental studies, and the food and pharmaceutical industries.
By understanding Beer’s Law, scientists can measure how much light a solution absorbs and relate that to the concentration of the dissolved substance. This principle forms the backbone of spectrophotometric analysis, a technique widely used to monitor chemical reactions and ensure quality control.
The Origin of Beer’s Law
Beer’s Law is named after August Beer, a German scientist who formulated the relationship between absorbance and concentration in 1852. Meanwhile, Johann Heinrich Lambert studied how light diminishes as it passes through a medium, leading to the combined Beer-Lambert Law. Today, this law is a fundamental tool in chemical laboratories and research centers worldwide.
The Science Behind Beer’s Law
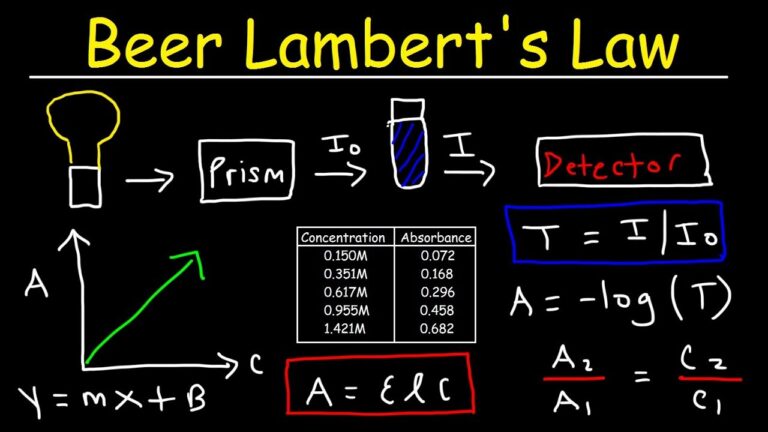
At its core, describes the linear relationship between the absorbance of light by a solution and the concentration of the solute. The standard mathematical expression is:
A=ε⋅c⋅lA = \varepsilon \cdot c \cdot l
Where:
-
A = Absorbance (dimensionless)
-
ε = Molar absorptivity (L·mol⁻¹·cm⁻¹)
-
c = Concentration of the solution (mol·L⁻¹)
-
l = Path length of the light through the solution (cm)
In essence, if you know two of the three parameters (absorbance, path length, and molar absorptivity), you can calculate the third—most commonly, the concentration of the solution.
How Beer’s Law Works
When light passes through a solution, molecules absorb some of the light energy. The amount of light absorbed depends on the concentration of the solute and the distance light travels through the solution. A spectrophotometer measures this absorbance, allowing scientists to determine unknown concentrations with high accuracy.
The beauty of lies in its simplicity and precision, making it a go-to method for chemical analysis.
Practical Applications of Beer’s Law
Understanding “What is is not limited to theory; it has significant real-world applications:
1. Chemical Analysis in Laboratories
Beer’s Law is widely used to determine the concentration of solutions in research laboratories, ensuring accurate results in experiments and quality control.
2. Environmental Monitoring
Scientists use this principle to measure pollutants in water and air. For example, detecting nitrate levels in rivers or measuring heavy metal contamination relies on .
3. Medical Diagnostics
Blood tests, enzyme activity measurements, and drug monitoring often employ spectrophotometric techniques based on Beer’s Law to ensure precise results.
4. Pharmaceutical Applications
Drug formulations require precise concentration measurements to maintain efficacy and safety, making a critical tool in the pharmaceutical industry.
5. Food and Beverage Quality Control
Beer’s Law is applied to measure sugar content, color intensity, and other chemical properties in beverages, guaranteeing consistent product quality.
Factors Influencing Beer’s Law
While is highly reliable, certain factors can affect its accuracy:
-
Solution Concentration: The law is most accurate for dilute solutions; high concentrations may lead to deviations.
-
Light Wavelength: Only monochromatic light should be used for precise measurements.
-
Chemical Interactions: Molecular interactions in the solution can affect absorbance.
-
Path Length: The thickness of the solution layer must remain consistent for accurate results.
By carefully controlling these variables, scientists can ensure precise measurements using .
Advantages of Beer’s Law
-
Quick and Accurate: Allows for fast concentration determination with high reliability.
-
Minimal Sample Requirement: Requires only a small volume of the solution.
-
Non-destructive: Preserves the sample for further analysis.
-
Versatile: Applicable across chemistry, biology, medicine, environmental science, and industry.
Limitations of Beer’s Law
-
Deviation at High Concentrations: Non-linear absorption can occur at high solute levels.
-
Scattering Effects: Particles in solution may scatter light, affecting measurements.
-
Chemical Interferences: Interactions between solutes can cause inaccuracies.
Despite these limitations, remains indispensable in scientific analysis and industry.
Conclusion
Understanding “What is Beer’s Law” is fundamental for anyone working with chemical solutions or spectrophotometry. This law provides a clear relationship between light absorbance and solute concentration, making it invaluable in laboratories, healthcare, environmental monitoring, and industry. By applying Beer’s Law correctly and considering its influencing factors, scientists can achieve precise, reliable measurements that are essential for research, quality control, and diagnostics.
FAQs
1. What is the primary use of Beer’s Law?
It is mainly used to determine the concentration of solutes in a solution by measuring light absorbance.
2. Can Beer’s Law be applied to colored and colorless solutions?
Yes, as long as the solution absorbs light at a specific wavelength, Beer’s Law can be applied.
3. Why is Beer’s Law only accurate for dilute solutions?
At high concentrations, molecular interactions and light scattering cause deviations from linearity.
4. How does path length affect Beer’s Law?
Absorbance is directly proportional to the path length; a longer path increases light absorption.
5. Which industries rely on Beer’s Law the most?
Pharmaceuticals, environmental science, food and beverage, medical diagnostics, and chemical research rely heavily on Beer’s Law.